20.09.2015
Lead-acid storage batteries are used in uninterruptible power supply systems (UPS), telecommunication, transmitter-receiver communication equipment, emergency power supply systems, emergency illumination and alarm systems, security and fire-prevention systems, electronic cash registers, off-line equipment etc., as a reserve power supply source.
It would seem, everything is abundantly clear, because there is an enormous number of brands, which produce batteries, and the best thing is to trust in a well-known (well-publicized) brand, because their batteries are better, but it appears that it’s not all that simple.
A battery is an electrochemical power source, the operation of which is based on using convertible electrochemical processes.
Principle of operation: electric energy accumulation inside the battery takes place when current passes through it from an outside source. This process named the battery charging is accompanied with electric energy converting into chemical. During the battery discharge there is the reverse converting of chemical energy into electrical.
DESIGN, MAIN PARAMETERS
Design. The simplest lead battery consists of a positive electrode, the active substance of which is lead dioxide (dark brown color), and a negative electrode, the active substance of which is spongy lead (grey color). If both electrodes are placed in a vessel with electrolyte (sulfuric acid solution in the distilled water), then there will be a potential difference between electrodes. Electrodes in modern batteries represent lead grids with active mass coated on them (plates). Initially the active mass of a grid represents powder of finely grained lead with addition of alloying materials, which provide plates with required technological properties.
When a load is connected to the battery, then lead plates with active mass jointly with electrolyte and the load form the closed circuit. Chemical reaction will start inside the battery, as a result of which the active mass of both electrodes will change their primary composition and the specific gravity of electrolyte will begin to reduce. In the total, the electric current will begin to flow in the circuit. Such process is named the battery discharge. When an outside current source is connected to the battery, the inverse process will start – charging. During charging, the active mass on plates regenerates its primary composition and the specific gravity of electrolyte grows.
One of main characteristics of a lead-acid battery is its capacity.
The capacity of a battery is the amount of electricity, which the full charged battery provides during its discharging up to the allowable end discharge voltage. The capacity of a battery is measured in ampere-hours and determined as a product of discharge current value (in amperes) by discharge time (in hours).
As the current provided by the battery is produced due to chemical reaction, the capacity of a battery will not be a permanent value.
The battery discharge capacity depends on a number of structural and technological parameters, as well as on the battery operating conditions. The most significant structural parameters are the amount of active mass and electrolyte, thickness and geometrical dimensions of battery electrodes. Main technological parameters, which influence on the battery capacity, are the formulation of active materials and their porosity. Operating parameters – the electrolyte temperature and discharge current strength – also render substantial influence on the discharge capacity.
A chemical reaction between active mass and electrolyte takes place on the surface of active mass particles, therefore it is made of a porous material, in order the material is saturated properly with electrolyte and its maximum volume will participate in the reaction.
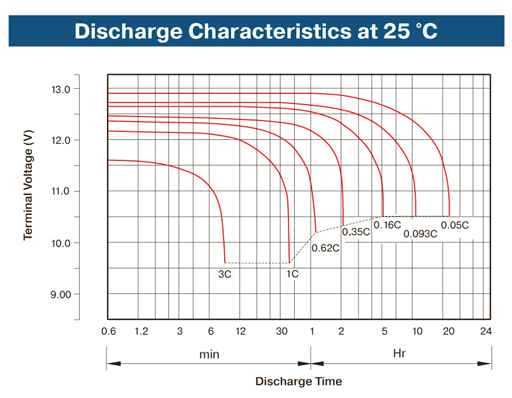
With rising discharge current value, the discharge of surface layers of active mass is accelerated in a greater degree, than depth layers. As the result, the increase of lead sulfate in pore throats takes place quicker than in the depth, and pores are clogged up with sulfate quicker than its internal layer is able to react (specific volume of lead sulfate is higher than specific volumes of lead and lead dioxide). Due to the electrolyte diffusion stoppage inside pores, the reaction in it is halted. Consequently, the higher discharge current, the less battery capacity. Discharge characteristics of SV12500 battery (C– 50 А•h, battery capacity) can be given as an example).
The rated capacity of a lead-acid battery is considered the capacity at the discharge during 20 hours, i.e. by the current 0.05С (C – the battery capacity in А•h).
In case the discharge current increases up to 50 A (1C), the battery will operate for 30 minutes (0.5 hour) and will provide to the load 50 × 0.5 = 25 А•h. The battery capacity with such discharge current is two times less.
TECHNOLOGIES
From the chemistry point of view, all processes in every lead-acid battery are similar. Distinctions in operating characteristics of batteries are conditioned by a design and applied technology. Car batteries usually cheaper than other batteries.
It is worth noting that car batteries are of little use as a reserve power supply source.
The design of a car battery envisages relatively large amount of free electrolyte (due to the operation features: powerful starter discharge currents, operation in winter). With such a design, it is difficult to provide some fixation of a package of plates.
ПWhen a battery is overdischarged, then the loosening of the active masses of positive electrode takes place, which in a starter car battery will lead to active mass sliding and loss of capacity. The discharge of car batteries below the residual voltage level 11.2 V is undesirable. Most battery-powered household appliances disconnect the battery, when the residual voltage level is 9.5 – 10.5 V.
Valve regulated lead-acid batteries (VRLA), which are manufactured according to two technologies, are more suitable for such devices as a reserve power supply source.
The first most widespread technology – Absorptive Glass Mat (AGM) is a subspecies of valve regulated lead-acid batteries, in which porous fiberglass filler-separator saturated with liquid electrolyte is used. Such a design enables to retain the electrolyte inside separator pores like a sponge.
The second, Gelled Electrolyte (GEL), or gel batteries (electrolyte in which is in the gel state) is a subspecies of the valve regulated lead-acid batteries, in which immobilization (immobility) of electrolyte is achieved by adding silicon compounds to sulfuric acid.
Plates in both subclasses are identical, but physical nature of recombination (due to which the impermeability is achieved) is different. AGM batteries are cheaper than gel batteries, they enable to develop high discharge and charge currents, they are less critical to charge conditions, do not afraid of momentary short circuit. This has been stipulated the wider application of AGM batteries. Gel batteries is reasonably to use there, where their advantage – bigger resource in the cyclic mode (gel fixes material of plates more effectively, reducing their wear during over discharging modes, therefore the cyclic resource of gel batteries is higher than of АGM) – is in demand (for example, in self-contained solar power supply systems). In many other devices, where the buffer recharging mode with rare discharges is used, gel batteries are used quite rarely.
SELECTION
It is worth noting that the real service life of batteries is determined by the maximum number of cycles of charge/discharge and under the real operation conditions it is rarely reach to the rating life 5-10 years (service life in years is a conventional degree).
It should be also realized that voltage 12 V on terminals does not guarantee in any way the availability of rated capacity in a battery, which means that it is impossible to define the battery health by voltage.
When purchasing a battery you should be governed by the manufacturer’s recommendations of the device for which it is intended.
For uninterruptible power supplies, which have relatively high output power and low off-line operation time (up to 30 minutes), batteries must be replaced only for the same type models (similar type, capacity and voltage).
When selecting batteries for long-lasting uninterruptible power supplies (for example, to provide power supply to gas boilers), you should be governed by the manufacturer’s recommendations of the device. It is obviously, the more battery capacity, the longer off-line operation time of UPS.
Current magnitude required for the battery charging is proportional to its capacity. Approximate ratio is 1 to 10. That is in order to charge a battery with capacity 100 А•h, the charge current approx. 10 А is required.
Most of UPS have built-in chargers designed to operate with batteries of certain capacity. The use of batteries with higher or lower capacity is undesirable.
All manufacturers specify the battery capacity, which is suitable for a certain UPS model. If UPS is able to use different batteries by capacity, the device must have a unit to control the charge current. It goes without saying that such UPS are much more expensive. But in any case, even such UPS have its own range by capacity of batteries being charged.
It should be also kept in mind that batteries have limited storage time; therefore, you have also to pay attention to a date of manufacture.
Lead-Acid Batteries
LEAD-ACID BATTERIES: PURPOSE, DEFINITION, APPLICATION AREA, PRINCIPLE OF OPERATIONLead-acid storage batteries are used in uninterruptible power supply systems (UPS), telecommunication, transmitter-receiver communication equipment, emergency power supply systems, emergency illumination and alarm systems, security and fire-prevention systems, electronic cash registers, off-line equipment etc., as a reserve power supply source.
It would seem, everything is abundantly clear, because there is an enormous number of brands, which produce batteries, and the best thing is to trust in a well-known (well-publicized) brand, because their batteries are better, but it appears that it’s not all that simple.
A battery is an electrochemical power source, the operation of which is based on using convertible electrochemical processes.

RL 12-50 lead-acid storage battery |
A battery is able to accumulate electric energy inside and to supply it to the external circuit as and when required.
Principle of operation: electric energy accumulation inside the battery takes place when current passes through it from an outside source. This process named the battery charging is accompanied with electric energy converting into chemical. During the battery discharge there is the reverse converting of chemical energy into electrical.
DESIGN, MAIN PARAMETERS
Design. The simplest lead battery consists of a positive electrode, the active substance of which is lead dioxide (dark brown color), and a negative electrode, the active substance of which is spongy lead (grey color). If both electrodes are placed in a vessel with electrolyte (sulfuric acid solution in the distilled water), then there will be a potential difference between electrodes. Electrodes in modern batteries represent lead grids with active mass coated on them (plates). Initially the active mass of a grid represents powder of finely grained lead with addition of alloying materials, which provide plates with required technological properties.
When a load is connected to the battery, then lead plates with active mass jointly with electrolyte and the load form the closed circuit. Chemical reaction will start inside the battery, as a result of which the active mass of both electrodes will change their primary composition and the specific gravity of electrolyte will begin to reduce. In the total, the electric current will begin to flow in the circuit. Such process is named the battery discharge. When an outside current source is connected to the battery, the inverse process will start – charging. During charging, the active mass on plates regenerates its primary composition and the specific gravity of electrolyte grows.
One of main characteristics of a lead-acid battery is its capacity.
The capacity of a battery is the amount of electricity, which the full charged battery provides during its discharging up to the allowable end discharge voltage. The capacity of a battery is measured in ampere-hours and determined as a product of discharge current value (in amperes) by discharge time (in hours).
As the current provided by the battery is produced due to chemical reaction, the capacity of a battery will not be a permanent value.
The battery discharge capacity depends on a number of structural and technological parameters, as well as on the battery operating conditions. The most significant structural parameters are the amount of active mass and electrolyte, thickness and geometrical dimensions of battery electrodes. Main technological parameters, which influence on the battery capacity, are the formulation of active materials and their porosity. Operating parameters – the electrolyte temperature and discharge current strength – also render substantial influence on the discharge capacity.
A chemical reaction between active mass and electrolyte takes place on the surface of active mass particles, therefore it is made of a porous material, in order the material is saturated properly with electrolyte and its maximum volume will participate in the reaction.
With rising discharge current value, the discharge of surface layers of active mass is accelerated in a greater degree, than depth layers. As the result, the increase of lead sulfate in pore throats takes place quicker than in the depth, and pores are clogged up with sulfate quicker than its internal layer is able to react (specific volume of lead sulfate is higher than specific volumes of lead and lead dioxide). Due to the electrolyte diffusion stoppage inside pores, the reaction in it is halted. Consequently, the higher discharge current, the less battery capacity. Discharge characteristics of SV12500 battery (C– 50 А•h, battery capacity) can be given as an example).
In this diagram, when the discharge current is equal to 0.05С (0.05 × 50 = 2.5 А, for this battery), the battery will discharge within 20 hours and will provide to the load 2.5 × 20 = 50 А•h (50 А•h – the rated battery capacity). The discharge current, for which the battery is designed and which is specified by a manufacturer, is named the rated capacity.
The rated capacity of a lead-acid battery is considered the capacity at the discharge during 20 hours, i.e. by the current 0.05С (C – the battery capacity in А•h).
In case the discharge current increases up to 50 A (1C), the battery will operate for 30 minutes (0.5 hour) and will provide to the load 50 × 0.5 = 25 А•h. The battery capacity with such discharge current is two times less.
TECHNOLOGIES
From the chemistry point of view, all processes in every lead-acid battery are similar. Distinctions in operating characteristics of batteries are conditioned by a design and applied technology. Car batteries usually cheaper than other batteries.
It is worth noting that car batteries are of little use as a reserve power supply source.
The design of a car battery envisages relatively large amount of free electrolyte (due to the operation features: powerful starter discharge currents, operation in winter). With such a design, it is difficult to provide some fixation of a package of plates.
ПWhen a battery is overdischarged, then the loosening of the active masses of positive electrode takes place, which in a starter car battery will lead to active mass sliding and loss of capacity. The discharge of car batteries below the residual voltage level 11.2 V is undesirable. Most battery-powered household appliances disconnect the battery, when the residual voltage level is 9.5 – 10.5 V.
Valve regulated lead-acid batteries (VRLA), which are manufactured according to two technologies, are more suitable for such devices as a reserve power supply source.
The first most widespread technology – Absorptive Glass Mat (AGM) is a subspecies of valve regulated lead-acid batteries, in which porous fiberglass filler-separator saturated with liquid electrolyte is used. Such a design enables to retain the electrolyte inside separator pores like a sponge.
The second, Gelled Electrolyte (GEL), or gel batteries (electrolyte in which is in the gel state) is a subspecies of the valve regulated lead-acid batteries, in which immobilization (immobility) of electrolyte is achieved by adding silicon compounds to sulfuric acid.
Plates in both subclasses are identical, but physical nature of recombination (due to which the impermeability is achieved) is different. AGM batteries are cheaper than gel batteries, they enable to develop high discharge and charge currents, they are less critical to charge conditions, do not afraid of momentary short circuit. This has been stipulated the wider application of AGM batteries. Gel batteries is reasonably to use there, where their advantage – bigger resource in the cyclic mode (gel fixes material of plates more effectively, reducing their wear during over discharging modes, therefore the cyclic resource of gel batteries is higher than of АGM) – is in demand (for example, in self-contained solar power supply systems). In many other devices, where the buffer recharging mode with rare discharges is used, gel batteries are used quite rarely.
SELECTION
It is worth noting that the real service life of batteries is determined by the maximum number of cycles of charge/discharge and under the real operation conditions it is rarely reach to the rating life 5-10 years (service life in years is a conventional degree).
It should be also realized that voltage 12 V on terminals does not guarantee in any way the availability of rated capacity in a battery, which means that it is impossible to define the battery health by voltage.
When purchasing a battery you should be governed by the manufacturer’s recommendations of the device for which it is intended.
For uninterruptible power supplies, which have relatively high output power and low off-line operation time (up to 30 minutes), batteries must be replaced only for the same type models (similar type, capacity and voltage).
When selecting batteries for long-lasting uninterruptible power supplies (for example, to provide power supply to gas boilers), you should be governed by the manufacturer’s recommendations of the device. It is obviously, the more battery capacity, the longer off-line operation time of UPS.
Current magnitude required for the battery charging is proportional to its capacity. Approximate ratio is 1 to 10. That is in order to charge a battery with capacity 100 А•h, the charge current approx. 10 А is required.
Most of UPS have built-in chargers designed to operate with batteries of certain capacity. The use of batteries with higher or lower capacity is undesirable.
All manufacturers specify the battery capacity, which is suitable for a certain UPS model. If UPS is able to use different batteries by capacity, the device must have a unit to control the charge current. It goes without saying that such UPS are much more expensive. But in any case, even such UPS have its own range by capacity of batteries being charged.
It should be also kept in mind that batteries have limited storage time; therefore, you have also to pay attention to a date of manufacture.

 zoom
zoom
